Contact Person:
Dipl.-Ing. Philip Tempel
Justus-von-Liebig Weg 6
18059 Rostock
Tel.: +49 381 / 498 - 9115
Fax: +49 381 / 498 - 9092
Email: philip.toellneruni-rostockde
Room: Complex building, room 16
Microfluidics and Simulation
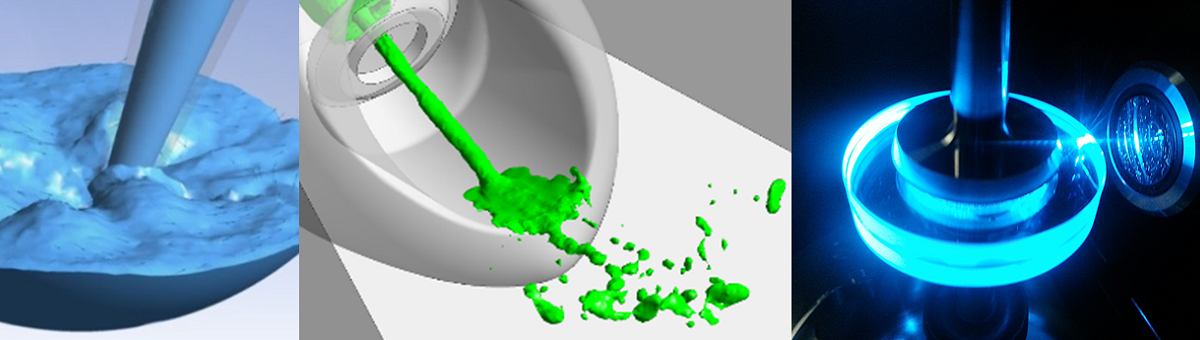
Microfluidics deals with issues relating to the handling, manipulation, and analysis of fluids where the fluid volumes are to be handled, and, therefore, the required dimensions of the microfluidic systems are very small. In addition to pure liquids and gases, multiphase fluid systems such as suspensions (especially cell suspensions) and emulsions are becoming increasingly important. Microfluidic systems are used in medicine and biotechnology, e.g., for rapid tests for substances or pathogens, in the chemical and pharmaceutical industries in the form of miniaturized and parallelized lab-on-a-chip systems and reservoirs loaded with active substances by inkjet printing, as well as in materials science for surface functionalization. The processes investigated in these systems include transportation, dosing, separation and filtration, mechanical loading and treatment with ultrasound, heat, and light (UV, visible, and infrared), droplet generation, and active ingredient loading by inkjet printing.
Due to the small fluid volumes in the range of a few microliters, sometimes only a few picoliters in modern applications, the space requirements and energy consumption for manipulating the systems are significantly reduced. In addition, effects and properties that play a subordinate role in the macroscopic world, such as capillary forces and mostly laminar instead of turbulent flows, come to the fore. As a result, microfluidic processes can be adjusted efficiently and precisely, especially under sterile conditions. In addition to various production technologies for the fabrication of prototypes of microfluidic assemblies, modern measuring methods for flow characterization (including a micro-PIV system) and measuring methods for determining various fluid properties (mass density, viscosity, and rheological behavior, contact angle, surface tension, refractive index, vapor pressure, etc.) are available. In addition to experimental investigations, the Chair of Microfluidics is also increasingly carrying out numerical flow simulations (CFD) to identify potential problems at an early stage of development.
Current Research projects
In close cooperation with Human Med AG and the Department of Cell Biology at Rostock University Medical Center, this joint project aims to develop a suitable drive for an innovative combination of vibration-assisted liposuction (PAL) and water-jet-assisted liposuction (WAL). One focus of the project is developing and applying novel test rigs to analyze the fluid mechanics of the generated water jet, the handpiece's vibration, and the device's sound emission. Another objective is to analyze the shear stress that acts on the fatty tissue in the new procedure. Investigations using numerical flow simulation (computational fluid dynamics simulation) accompany the experimental analysis to determine critical process limits.
A further aim is to develop a training model for users of vibration-assisted liposuction, water-jet-assisted liposuction, and related procedures. The target groups of the training model are, for example, doctors and other medical staff. The success of treatment using liposuction procedures depends very much on the user's level of experience with these procedures. The new training model to be developed is intended to allow users to gain experience in the practical application of WAL,
PAL, and related procedures at an early stage and without risk to patients. The training model should represent the anatomical conditions of certain human body regions as realistically as possible. To implement such a training model, the aim is to develop tissue analogs for fatty tissue, skin tissue, and bony structures, as well as suitable 3D printing-based manufacturing processes. The tissue analogs and manufacturing processes are to be developed to implement realistic training models for different body regions.
Person in charge: Dr.-Ing. Erik Westphal, M.Sc. Florian Neukirch
Project duration: 10/2023 – 09/2026
Funded by: Ministerium für Wirtschaft, Infrastruktur, Tourismus und Arbeit M-V. Das Projekt wird im Rahmen des EFRE Programms 2021 bis 2027 des Landes Mecklenburg-Vorpommern aus Mitteln des Europäischen Fonds für regionale Entwicklung der Europäischen Union und des Landes Mecklenburg-Vorpommern gefördert.
Partners:
- Human Med AG, Schwerin
- Arbeitsbereich Zellbiologie, Universitätsmedizin Rostock
SFB 1270 Elaine - Subproject C02: Electrical and mechanical stimulation of cartilage
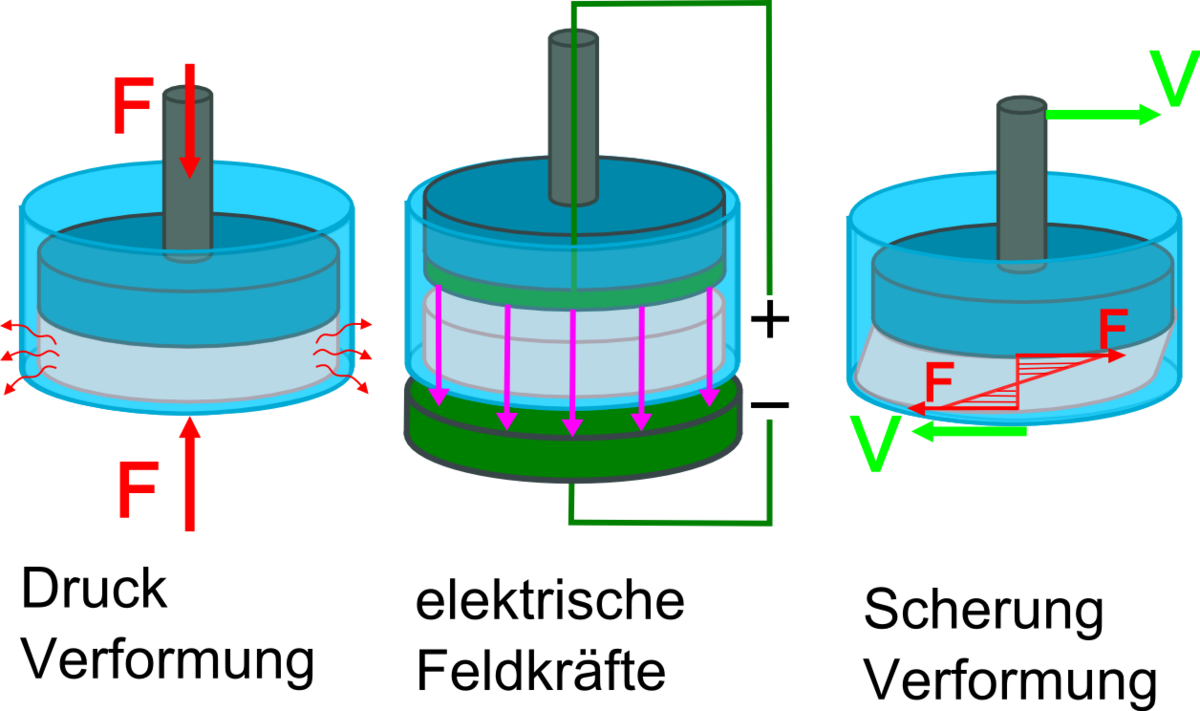
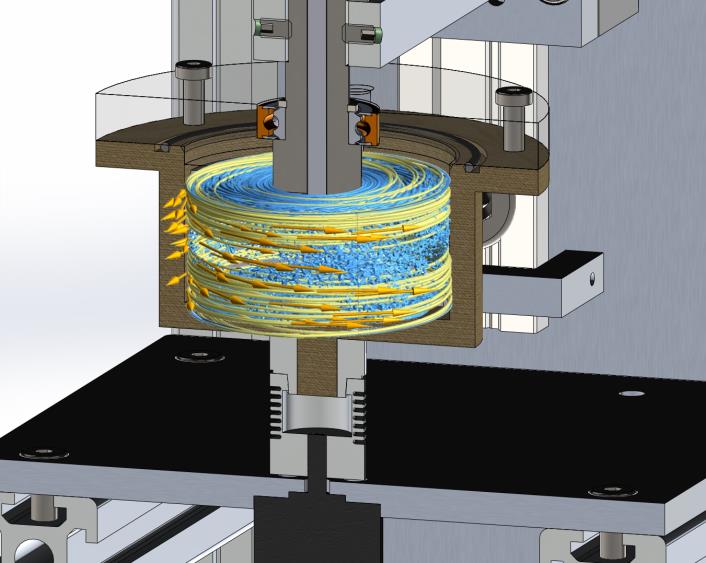
The subproject (C02) of the SFB Elaine aims to analyze the influence of electrical and mechanical stimulation on the chondrogenic differentiation of human cartilage cells and mesenchymal stem cells. This sub-project is being carried out in collaboration with the Research Laboratory for Biomechanics and Implant Technology at Rostock University Medical Center. The scaffolds developed in subproject B01 will be used as a scaffold for the cultivation of cartilage cells. A 3D stimulation chamber is being developed specifically for experimental investigations, which makes it possible to apply electrical or mechanical stimulation or even a combination of both types of stimulation. Key issues include determining the configuration of the electrodes and implementing the mechanical actuators for shear and pressure loading of the cells. In addition, a protocol for electrical and mechanical stimulation is being developed, leading to optimized proliferation and differentiation of the cartilage and mesenchymal stem cells. In addition, a comprehensive "multiphysics" simulation model is to be created that maps the stimulation processes (electrical and mechanical) and the effect on the cells (e.g., shear stress) and contributes to a better understanding of the mechanical and bioelectromagnetic stimulation processes. The long-term aim is to optimize the ex vivo 3D cultivation of cartilage cells and mesenchymal stem cells using electrical and mechanical stimulation in autologous cartilage cell implantation using a collagen- and hydrogel-based 3D implant (scaffolds).
Person in charge:Nada Abroug M.Sc.
Project duration: 07/2017 - 06/2025
Analysis, simulation, and modification of the anisotropic properties of fiber-reinforced adhesives
In the ASiMof project, the Chair of Microfluidics is developing a CFD simulation model to determine the fibre orientation during the application of a selected adhesive and then simulatively optimizes the bonding process with the aid of this model about fibre orientation. The fiber alignment determined by CFD simulation is to be compared experimentally with samples bonded at the Chair of Production Engineering, which are examined by micro-CT at the Institute of Joining and Welding Technology at the TU Braunschweig.
The CFD model developed should make it possible to predict the fiber orientation, which can be specifically influenced with the help of various influencing variables (shape of the application nozzle, application speed, joining process). By simulating the flow behavior during application and joining, influencing variables resulting from the adhesive formulation (fiber length, fiber thickness) are also considered. The orientation of the short fibers is to be determined using the orientation tensor, which is to be implemented in the CFD solver ANSYS FLUENT during the project
Person in charge:Muhammad Asad Yamin, M.Sc.
Project duration: 09/2022 - 10/2024
Funded by: AiF – Forschungsnetzwerk Mittelstand, IGF - Industrielle Gemeinschaftsforschung
Partners:
- Universität Rostock, Lehrstuhl für Fertigungstechnik, Prof. Dr.-Ing. Wilko Flügge
- TU Braunschweig, Institut für Füge- und Schweißtechnik IFS, Prof. Dr.-Ing. Klaus Dilger
Completed research projects
The research project aims to develop a new, modular electrohydraulic pump system that allows energy recovery and resource-saving use in four quadrant operations. The aim is to increase the pressure and speed range with the smallest possible installation space and the lowest possible noise emissions.
The sub-project of the Chair of Microfluidics is concerned with maximizing efficiency by optimizing flow control. Due to the four-quadrant operation, the flow control in the system poses a major challenge, particularly at high speeds and pressures. To increase efficiency, the flow control must be optimized in relation to the operating mode. As part of the project, various geometric designs will be analyzed iteratively using flow simulation and evaluated about optimized flow routing. In particular, cavitation-critical and pressure loss-critical points must be detected and minimized through suitable geometry changes.
Person in charge:Pedram Azizi M.Sc.
Project duration: 06/2021 - 10/2023
Funded by: Ministerium für Wirtschaft, Arbeit und Gesundheit (M-V), Operationelles Programm für den Europäischen Fonds für regionale Entwicklung in Mecklenburg-Vorpommern in der Förderperiode 2014 bis 2020 (EFRE-OP M-V)
Partners:
- Hydraulik Nord Technologies GmbH, Parchim
- Universität Rostock; Lehrstuhl für Getriebe- und Antriebstechnik
- Fraunhofer IGP, Rostock
As part of the joint project Actiheal, innovative technologies for activating and applying tissues and cells to treat chronic wounds are being developed.
The aim of the project is the biomodulation and clinical application of regenerative cells of the stromal vascular fraction for the treatment of chronic wounds, which occur, for example, in diabetic patients (diabetic foot). These treatments should make it possible to noticeably improve patients' quality of life and prevent the complications typical of diabetes.
For this purpose, mesenchymal stem cells are extracted from human adipose tissue (adipose derived mesenchymal stem cells, adMSC). The stem cells are then irradiated using near-infrared to infrared light (low-level-light-therapy, LLLT) or perfused with cold atmospheric plasma and applied autologously. The biomodulation processes should increase the proliferation (multiplication of cells through cell division) of the stem cells and thus improve wound healing. In this joint project, the Chair of Microfluidics is responsible for developing, producing, and optimizing microchannel cassettes for photobiomodulation and plasma biomodulation of cell suspensions and microtissue clusters from human adipose tissue. The cassette prototypes are produced using additive manufacturing processes of biocompatible materials, enabling rapid optimization.
Person in charge: Mario Thürling M.Sc. (Elternzeit); Florian Neukirch M.Sc.
Project duration: 09/2019 bis 09/2023
Funded by: Ministerium für Wirtschaft, Bau und Tourismus (M-V), Operationelles Programm für den Europäischen Fonds für regionale Entwicklung in Mecklenburg-Vorpommern in der Förderperiode 2014 bis 2020 (EFRE-OP M-V)
Partners:
- Human Med AG, Schwerin
- Arbeitsbereich Zellbiologie, Universitätsmedizin Rostock
- Kompetenzzentrum Diabetes, Klinikum Karlsburg
Creation of a development environment for active steering systems
A new type of active steering system is to be developed as part of the overall project, which can be adapted to various vehicle topologies thanks to its modularity. The work is being carried out by several partners in sub-projects.
The sub-project at the Chair of Microfluidics aims to develop a steering unit in the form of a valve block further using numerical flow simulation and additive manufacturing.
Person in charge:Oliver Schmiedl M.Sc.
Project duration: 01/2018 - 12/2022
HOGEMA joint project
As part of the HOGEMA joint project, hydrostatic high-pressure technology (HHD) is to be optimized and made usable for the processing of various types of tissue from supporting (bone, cartilage) and connective (fascia) tissue for the production of allografts and tissue model systems. The aim is to enable the treatment of tissue defects with allogenic transplant tissue.
The hydrostatic high-pressure technology (HHD) is intended to devitalize the tissue quickly and gently without negatively impacting its structural properties. The Chair of Microfluidics is responsible for developing a new type of irrigation chamber in close cooperation with the IPT Wismar. This is used for the semi-automated, gentle, reproducible, and time-saving cleaning of HHD-treated allografts of various tissues. Microbiological contamination will be excluded, and tissue residues consisting of blood, connective tissue residues, bone marrow, muscle tissue, and cell residues will be removed. Combined with the HHD treatment, the rinsing chamber addresses the current deficit of a less standardized and time- and process-intensive treatment of allografts with chemically aggressive media. The Chair of Microfluidics is setting up test rigs for selected mechanical cleaning principles, and new scientific findings on optimum performance parameters are being developed. This is done hand in hand with the clinical partners, who provide allograft samples and contribute their expertise (e.g., histological staining) for evaluation. Iteratively, functional models of an irrigation chamber will be created using state-of-the-art manufacturing methods (e.g., additive manufacturing), which will be made available to the partners during the project. In addition, a test environment for the functional models will be established, allowing the validation of technical operating parameters and the use of allograft samples.
Within the HOGEMA joint project, the tissues are to be extensively characterized in cell culture and animal studies after processing to test their feasibility for later clinical application. In addition, the project aims to provide devitalized tissue for developing and establishing physiologically similar model systems, which will subsequently be used instead of animal models for various basic research questions.
Person in charge:Dr.-Ing. Christoph Drobek
Project duration: 01.12.2018 - 30.06.2022
Collaborative project: Development of a system for automated cell fractionation from human adipose tissue for novel regenerative applications and therapies (ARENA)
The aim of the sub-project is to support the development of a new system for obtaining regenerative cells from lipoaspirate by developing a new method for separating large quantities of stem cells from human adipose tissue. The aim is to dispense with tissue dissociation with collagenases completely. To this end, experiments are to be developed and carried out in cooperation with the Department of Cell Biology at Rostock University Medical Center, in which various active principles will be
investigated. As the project partners assume that mechanical properties have a major influence on stable cell separation, a better scientific understanding of the mechanical properties of fat and stem cells is to be gained in addition to the purely experimental development. The ARENA project is funded by the "European Regional Development Fund" (ERDF) from the European Structural Funds of the European Union."
Person in charge: Dr.-Ing. Christoph Drobek
Funded by: Europäischen Fonds für regionale Entwicklung (EFRE)
Project duration: 10/2015 - 11/2018
Partrners: HUMAN MED AG, Schwerin; Zellbiologie, Universitätsmedizin
Experimental and numerical methods for flow analysis in centifluidic systems for allergy diagnostics
Due to their complexity, centifluidic diagnostics and medical technology systems often have to be considered at the system level. Only at this level can the system's overall behavior, consisting of interactions between different physical effects, be described. The rapid test system for allergy diagnostics (FastCheckPOC 20) developed by DST and already successfully established on the market could previously only be used by performing complex manual tests. The aim is to develop a concept for the automated execution of the various work steps. Due to the current problems of the project partner in the development of automated execution about the flow behavior in a sub-module of the test execution, the reagent module, the work within this project focuses on the modeling, numerical simulation, and experimental analysis of the fluid dynamics in this module. New geometric approaches for the channel structure in the reagent module are being developed. Based on these approaches, several parameter and filling studies with different channel depths and volume flows are carried out and subsequently validated with the aid of numerical flow simulation (CFD). The insights into the flow behavior gained in this way flow directly into the development of the automated test execution.
Person in charge: Dipl.-Ing. Manuel Dethloff
Project duration: 03/2015 - 12/2017
Funded by: Bundesministerium für Wirtschaft und Energie (BMWi)
Partners: DST Diagnostische Systeme & Technologien GmbH, Schwerin